Abstract
Background Chronic myeloid leukaemia (CML) is a clonal myeloproliferative disorder, hallmarked by the BCR-ABL fusion gene which encodes for a constitutively activated BCR/ABL tyrosine-kinase, effectively treated with Tyrosine-Kinase Inhibitors (TKIs). The first line TKI Imatinib (IM) can obtain a high cumulative CCyR; compared to IM, deeper and faster molecular responses can be obtained in newly diagnosed CML patients with the second generation TKIs, Nilotinib (NIL) and Dasatinib. Despite the three TKIs have been evaluable as first line therapy for a long time, our experience largely concern the second generation TKIs after IM but not evidence are reported regarding IM after second generation TKIs.
On behalf of the Rete Ematologica Lombarda (REL) we designed the PhilosoPhi34 study (EudraCT: 2012-005062-34). This study was divided into three consecutive phases. An initial recruitment phase, a "core" phase of treatment lasting for 12 months (mos) and an "observational" phase lasting for an additional period of 24 mos. NIL 300 mg BID standard dose was administered for one year ("core" phase). At the end of this "core" phase of the trial, during the "observational" period, treatment options were up to the Investigator's choice (i.e. any TKI approved as first line treatment could be used), based on individual patient evaluation.
Furthermore, fluctuations of BCR/ABL ratio are known, in particular during the two first years of CML treatment, characterized by a higher risk of disease progression or relapse.
Aims Despite this consideration, our purpose is to verify time courses of Molecular Response (MR) IS in the IM treated patients, during the "observational" phase of the PhilosoPhi34 study.
Methods We evaluate the PhilosoPhi34 database. MR IS is reported at 3, 6 and 12 mos of NIL treatment ("core" phase) and every 6 mos in the "observational" phase. Until now, the "observational" phase is ongoing and the database is unlocked.
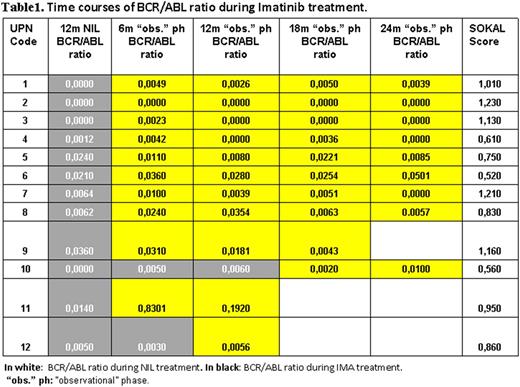
Results Among the 74 reported pts who started the "observational" phase, with at minimum a CCyR, 62 pts maintained NIL, 10 pts switched to IM and 2 pts switched to other TKI. Other two pts switched to IM after 18 and 24 months of NIL treatment (i.e. +6 mos and +12 mos of the "observational" phase, respectively). Among the 62 pts that maintained NIL, 33 showed a MR ≥ 4.0 IS and 20 showed a MR 3.0 IS. In the NIL group 15/62 pts showed an increase of the BCR/ABL ratio after 6 mos of the "observational" phase, but only 5/33 (15%) pts lost MR4.0 IS and 1/20 (5%) pts transient lost MR3.0 IS. Overall, 1/62 pts failed at 26 mos of treatment (+14 mos of "observational" phase) harbouring a resistant mutation. All the other NIL treated pts, despite molecular fluctuations, maintained or improved MR over time. Among the 10 pts that switched to IM after 12 mos of NIL treatment, 6 were in MR ≥ 4.0 IS and 4 in MR3.0 IS. Of them, 7/10 pts showed an increase of the BCR/ABL ratio but only 1/6 (16,6%) pts lost MR4.0 IS and 1/4 (25%) pts lost MR3.0 (Table1). No pts failed treatment. Of note, the probability to lose MR≥ 4.0 IS or MR 3.0 IS between NIL treated pts vs IM treated pts proved not significant with the Fisher's test (p: 0.43 and p: 0.28, respectively).
Conclusion As expected, our preliminary data show fluctuation of BCR/ABL ratio and of MR IS. IM treated pts present initial molecular fluctuations statistically similar to the fluctuation showed by NIL treated pts evaluated at the same time point. Despite fluctuations, MR IS is maintained or improved, over time. More data are necessary to confirm these exploratory results. Pointing out the possible adverse events occurring during long-term NIL treatment and the percentage of CML pts with vascular or metabolic disease at diagnosis, we consider a short-term treatment with NIL before IM a new possible strategy for CML cure.
Orlandi: Novartis: Speakers Bureau; Bristol-Myers Squibb: Speakers Bureau; Incyte: Speakers Bureau. Iurlo: Pfizer: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria; Novartis: Consultancy, Honoraria. Rossi: Sanofi: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Teva: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.